Mixture is the combination of two or more pure substances where each substance retains its own identity. It is collection of dissimilar particles that will not undergo a chemical reaction.
A mixture has variable combinations
Example: alcohol-water mixture, both co-exist together.
A mixture can be either homogeneous or heterogeneous
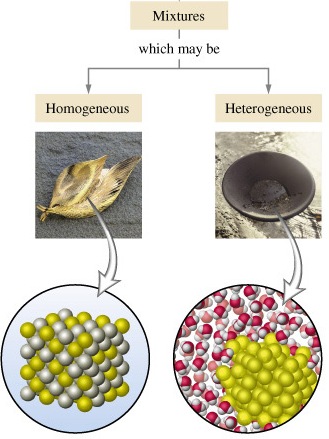
1. It has uniform composition
2. The particles are uniformly distributed
Example of Homogeneous mixture: alcohol-water mixture we call this homogeneous mixture as a solution, Sugar and water, Air is a homogeneous mixture of gases
Heterogeneous mixture
1. It has a non-uniform composition
2. Particles involved are not uniformly distributed
Example of Heterogeneous mixture: sea water, concrete, a mixture of salt water and sand.
Learn more: Elements, Compounds vs Mixtures
A mixture has variable combinations
Example: alcohol-water mixture, both co-exist together.
A mixture can be either homogeneous or heterogeneous

Homogeneous Mixture vs Heterogeneous Mixture
Homogeneous mixture 1. It has uniform composition
2. The particles are uniformly distributed
Example of Homogeneous mixture: alcohol-water mixture we call this homogeneous mixture as a solution, Sugar and water, Air is a homogeneous mixture of gases
Heterogeneous mixture
1. It has a non-uniform composition
2. Particles involved are not uniformly distributed
Example of Heterogeneous mixture: sea water, concrete, a mixture of salt water and sand.
Learn more: Elements, Compounds vs Mixtures
wow!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!
ReplyDeleteok
ReplyDeletegood
ReplyDeleteAbe buskar
ReplyDeleteReally helpful sir thank you.
ReplyDeleteOk
ReplyDeleteOk
ReplyDeleteThanks! This helped me with my science class!
ReplyDeleteThanks! This helped me with my science class!
ReplyDeletetgjrsfdnjd
ReplyDeleteWorst answer ever seen in the world
ReplyDeleteexcellent
ReplyDeleteBull shit
ReplyDeletePeace out nerds
OK ,😐😑🤔 need improvement
ReplyDeletewas not useful
ReplyDeletelol this is better https://www.thoughtco.com/heterogeneous-and-homogeneous-mixtures-606106
ReplyDeleteIt should have more differences
ReplyDeleteGod ek hi bar mein samajh as gya
ReplyDeleteGreat
ReplyDeleteSooooower
ReplyDeleteTrue
ReplyDeleteObviously
ReplyDeleteThere are very less differences
ReplyDelete��
ReplyDeleteWowwwwwwwww
ReplyDeleteJust fine there is one more point of difference between them .
ReplyDeletePost a Comment
We Love to hear from U :) Leave us a Comment to improve this site
Thanks for Visiting.....