Electrolytes are of two types: Strong electrolytes and
Weak electrolytes
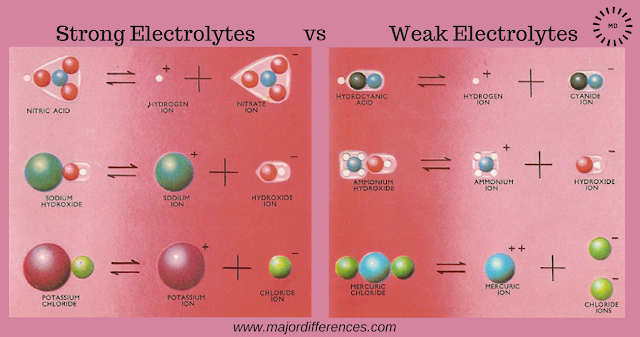
Strong electrolytes: These are substances which
dissociate almost completely in solution and hence conduct electricity to a
large extent.
Example of Strong
electrolytes: Strong acids (HNO3, H2SO4 etc,
strong bases (NaOH, KOH) etc and salts (KCl, NaCl) etc.
Weak electrolytes:These are substances which undergo
only slight dissociation in solution and
hence conduct electricity to a small extent.
Example of Weak electrolytes:
Weak acids(CH3COOH, H2CO3, HCN etc) and weak
bases (NH4OH, Ca(OH)2 etc.
Strong electrolytes vs Weak electrolytes
Strong electrolytes
|
Weak electrolytes
|
1. Completely dissociated at moderate concentrations
|
Not completely
dissociated at moderate concentrations |
2. Conductance increases with dilution but the increase is only
slight.
|
Conductance increases rapidly with dilution especially near
infinite dilution
|
3. There are strong interionic attraction at moderate concentrations.
|
Interionic attractions are not strong even at higher
concentrations
|
The λm vs √c plot is not linear even at low concentrations.
Here Lm is λm Variations of molar conductance with concentration |

Post a Comment
We Love to hear from U :) Leave us a Comment to improve this site
Thanks for Visiting.....